African Swine Fever (ASF)
Research / Protocols / Videos
PhD Thesis / Indexed publications / Outreach publications
African Swine Fever (ASF) is currently the biggest challenge for the pig sector globally and the biggest current problem in animal health. It is an infectious disease caused by the ASF virus that affects swine, both domestic and wild boar. Due to its important economic and animal health implications, it is on the list of notifiable diseases of the World Organization for Animal Health (OIE).
Its current situation is of great concern worldwide due to its extensive expansion in recent years, currently affecting four continents where more than 78% of the global swine population lives. ASF has not only maintained in many African countries, it has also advanced in countries on the European continent and has affected the Asian continent and islands close to Australia for the first time. In Asia, it has made its way through the largest pig producer, China, which is home to nearly half of the global pig population. During 2019, the number of domestic pigs slaughtered due to ASF was ten times greater than in 2007 and five times greater than in 2018 (Table 1).
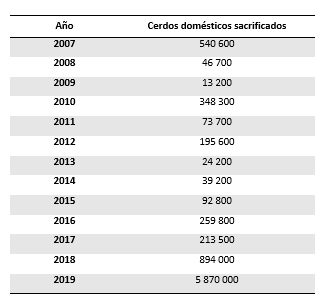
Tabla 1. Total number of domestic pigs slaughtered per year due to African swine fever (OIE, FAO).
The lack of vaccines and treatments available to fight against ASFV infection is one of the reasons for its great geographical expansion without control. In this sense, the control of ASF is based on the implementation of strict sanitary measures. However, sometimes, implementing the biosecurity measures necessary to stop the spread implies changes in traditional practices and ancestral customs, which represents a real challenge for health workers.
In this section you can find all the information on the epidemiology of ASF, its history, its current situation and the new research challenges, as well as regular updates by sending new communications.
ASF History
ASF was first described in Kenya in 1921 by Montgomery. It was described as a high lethality disease (99%) in recently imported European pigs. In the following decades, its presence was observed in several countries in sub-Saharan Africa.
First occurrence of ASF outside African continent occurred in Portugal in 1957, on a domestic pig farm near Lisbon. However, this outbreak was quickly controlled after the slaughter of more than 10 000 domestic pigs. After an epidemiological silence of three years, in 1960 the disease reappeared in Portugal, rapidly spreading to the whole Iberian Peninsula. Since then, ASF remained present in the Iberian Peninsula for approximately 35 years, until its eradication was achieved in 1994 in Portugal and 1995 in Spain, consequent to great human and economic efforts.
During these years of ASF in the Iberian Peninsula, several European and American countries suffered outbreaks of ASF (Figure 1), mainly caused by movement of contaminated meat products. However, these outbreaks were eradicated except in the island of Sardinia, where the disease remains endemic since 1978.
Currently, the island of Sardinia has been more than a year without positive notifications in domestic pigs or wild boar, the application to declare themselves an ASF-free country is ongoing.
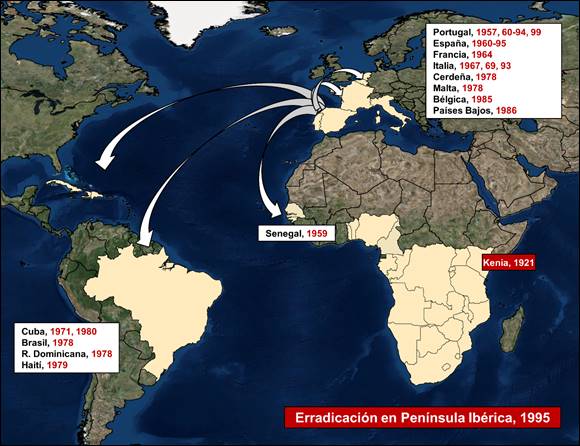
Figure 1. ASF epidemiology from 1957 to 1995.
Since the eradication of ASF in Spain in 1995, with the exception of Sardinia and an isolated outbreak in 1999 in Portugal, the disease remained confined to the African continent. In the last decade, ASF became very important in Africa, affecting a large number of countries previously free of disease, spreading to West and Central Africa, with a consequent increase in circulating virus and hence contaminated meat products.
This fact may have contributed to the most important epidemiologic change of ASF in recent years. In 2007, there was a great jump of the disease, with the reentry of ASF virus on the European continent, this time, by the Caucasus region. First outbreaks of ASF occurred in Georgia, near the port of Poti. Since then, ASFV has been spreading uncontrollably into new territories.
ASF current situation
Currently, ASF is present in four continents (Africa, Europe, Asia and Oceania), exceeded any historical scenario, where different epidemiological patterns are observed (Figure 2). The study of different epidemiological patterns is essential for an effective approach to the design and implementation of prevention and control measures. The different epidemiological scenarios currently observed are detailed below.
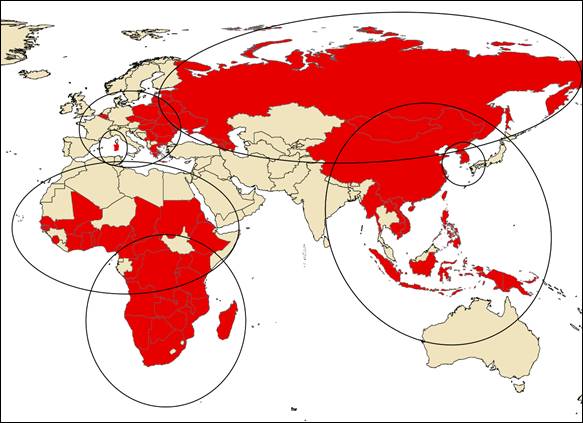
Figure 2. Current epidemiological scenarios of ASF.
Map
African swine fever OIE notifications.

African Swine Fever in Africa
In Africa, the swine population has doubled over the past three decades, leading to an increase in the number of notifications and regions affected by ASF. Currently, a large part of sub-Saharan countries are endemically affected by this disease. The 24 different genotypes of the ASF virus described to date have been observed in this territory, with the greatest variability being found in the eastern part of the continent.
In South East Africa is found a very complex epidemiological cycle characterized by the coexistence of wild swine, such as the bushpig (Potamochoerus larvatus), the warthog (Phacochoerus africanus) and the giant forest hog (Hylochoerus meinertzhageni), soft ticks of the O. moubata complex, and domestic pigs. Wild swine in South East Africa have the particularity of being tolerant to the development of the disease, acting as reservoirs and a permanent source of the virus. In addition, the ASF virus is able to replicate in the ticks of the O. moubata complex, which can act as vectors in the transmission of the virus between wild swine and domestic pigs. The participation of wild hosts, both swine and ticks, makes their eradication extremely difficult.
The situation is different in West Africa, where wild hosts are not present, being the domestic pig the only host involved. In such a way the control and eradication in West Africa may seem easier, however, it also represents a major health challenge. This is because the pig sector is affected by socio-economic factors that limit the producers' ability to implement the necessary control measures for better management of the disease.
African Swine Fever in Europe
In Europe, since its re-introduction in 2007 through Georgia, the ASF has been advancing uncontrollably. In the first place, the disease began its expansion to the north, affecting in the same year Armenia, Azerbaijan and the Russian Federation. Since the Russian Federation, the ASF has affected countries such as Ukraine (2012) and Belarus (2013).
Subsequently, in 2014, the disease entered the European Union (EU) through Lithuania, Poland, Latvia and Estonia. In 2016, nine other European countries have been affected such as Moldova (2016), Romania (2017), Czech Republic (2017), Bulgaria (2018), Hungary (2018), Belgium (2018), Slovakia (2019) , Serbia (2019) and recently Greece (2020) (Figure 3). This situation represents a great risk for neighboring countries such as France, Germany and Spain, the latter two are the largest pig producers in the European Union, so the notification of ASF in these countries could imply considerable economic losses.
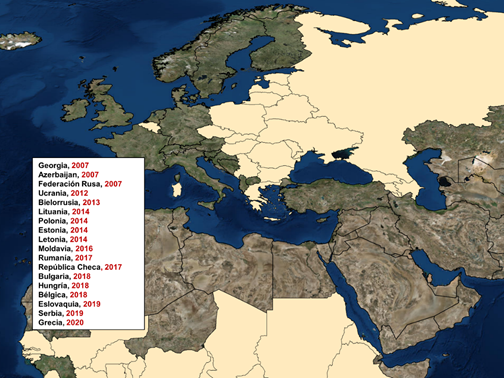
Figure 3. ASF epidemiology in Europe from 2007 to April 2020.
In Europe both domestic pigs and wild boar have been affected, the latter has shown to play a very important role in the persistence and circulation of the virus in the continent. In contrast to wild swine in Africa, Eurasian wild boar is susceptible to the development of the disease, presenting a clinical picture very similar to that observed in domestic pigs. The high abundance of the wild boar population in Europe determines very important aspects of the transmissibility of infectious diseases.
Within Europe, two different epidemiological scenarios have been observed depending on the predominant type of host affected. On the one hand, there are the affected countries of the European Union (with the exception of Romania, Bulgaria, Slovakia and Greece), where more than 90% of the notified cases are attributed to wild boar, with sporadic outbreaks in domestic pig farms. However, in Eastern Europe, including countries such as Russia, Ukraine, Belarus, Moldova and Romania, ASF mostly affects domestic pig farms and to a lesser extent wild boar. This difference can be explained by the fact that these latter countries have a greater number of family or backyard farms. This type of production system has low levels of biosecurity and are usually located in areas where wild boar lives, which increases the risk of transmission of the virus to domestic pigs. In addition, it is possible that in this type of farms feeding pigs with food waste is practiced. This activity has historically been one of the main routes of transmission of the ASF virus and is currently banned in the European Union.
The island of Sardinia, where ASF has been endemic since 1978, in recent years has achieved a considerable decrease in the number of notifications of the disease, both in domestic pigs and in wild boar. In this way, the island of Sardinia is close to being declared an ASF-free area. This has been possible after enormous efforts of epidemiological studies, of the administration and farms, being the capture and removal of the free-ranging pigs one of the most effective measures.
African Swine Fever in Asia
ASF was detected for the first time in Asia in August 2018, through China, at a domestic pig farm located in Shenyang City. The likely sources of introduction, based on epidemiological surveys, were the importation of piglets from infected areas and swill feeding. Genetic studies of the virus isolated showed similarities with those circulating in Europe.
The spread of ASFV throughout China has been considerably rapid compared to other epidemiological scenarios. This rapid spread has also been reflected in the cross-border advance, affecting 11 other Asian countries in 2019 (Mongolia, Vietnam, Cambodia, Hong Kong, North Korea, Laos, Myanmar, the Philippines, South Korea, Timor-Leste and Indonesia) (Figure 4). Recently, in March 2020, Papua New Guinea has confirmed the presence of ASFV in its territory, increasing concern in Australia due to the few hundred kilometers that separate its coasts.
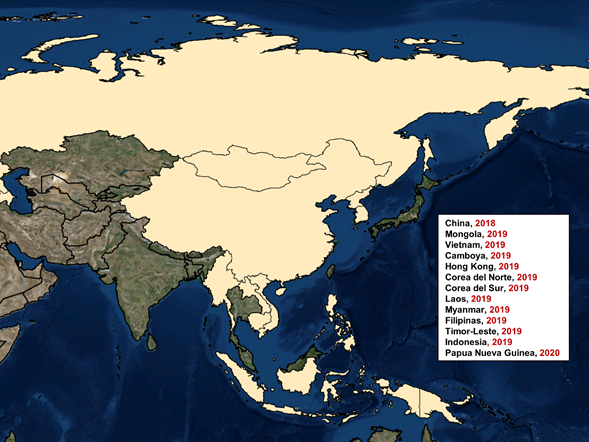
Figure 4. ASF epidemiology in Asia from 2018 to April 2020.
Some of the factors that are facilitating the spread of the virus in this continent are i) the lack of experience in the detection, management and control of ASF; ii) the characteristics of its pig sector, since there is a high number of backyard farms; and iii) the performance of traditional risk practices, such as swill feeding or even the use of blood or blood derivatives as a food supplement. With the exception of South Korea, where numerous cases have been reported in wild boar, most of the cases notified in Asian countries have been reported in domestic pigs.
Despite the control and prevention measures taken so far, ASFV has continued to advance to the continent, causing the death of at least 6 million animals. China is the world's largest pig producer, with more than 400 million pigs. Therefore, this situation is leading to serious economic and food supply problems, even affecting international trade due to the increase in price of pork.
ASF new challenges in research
In view of the current situation, it is necessary to reconsider and improve the strategies and programs for control and prevention against ASF. Among the great challenges of ASF control, highlights the lack of available vaccine, as well as the existence of different epidemiological scenarios.
For that reason, much of the research studies in ASF are focusing on the development of a safe and effective vaccine. The development of a vaccine against ASFV has been hindered throughout history by the great complexity of the virus, the lack of knowledge of the virus-host interactions, the absence of a neutralizing immune response and certain technical difficulties such as adaptation of the virus to a stable cell line. Currently, our SUAT-UCM group (www.sanidadanimal.info) is coordinating the VACDIVA project (www.vacdiva.eu) (nº 862874, H2020), endowed with 10 million euros, in order to expand knowledge in this area and to be able to get a safe and effective DIVA vaccine, both for domestic pigs and wild boar.
On the other hand, it is important to continue epidemiological studies aimed at identifying the risk factors in the affected regions and the potential routes of introduction in free countries. Because it is necessary to adapt the control and eradication programs to each of the epidemiological scenarios, incorporating their cultural and sociological characteristics, as well as reinforcing prevention measures in free scenarios. Training veterinarians and farmers on ASF is essential to prevent further spread of the disease.
In this way, an effective and safe vaccine together with the establishment of personalized control and eradication plans for each of the epidemiological scenarios could be the solution to stop the continuous spread of ASFV.
Protocols
- Serological diagnosis of ASF by ELISA commercial kit
- Serological diagnosis of ASF by OIE ELISA indirect test
- Detection of ASFV by conventional polypmerase chain reaction (PCR)
- Detection of ASFV by Real Time polymerase chain reaction (PCR)
- Genotyping of ASFV isolates
- Pen side test for ASF antibody detection
Videos
Interviews
ASF clinical presentation
Protect your farm against ASF
ASF The disease
PhD Thesis
- Avances en la monitorización y el control de la Peste Porcina Africana en la interfaz jabalí-cerdo doméstico
Estefanía Cadenas Fernández
Universidad Complutense de Madrid (realización en curso)
- Nuevas estrategias para el control y erradicación de la peste porcina africana
Cristina Jurado Díaz
Centro de Vigilancia Sanitaria Veterinaria (VISAVET). Universidad Complutense de Madrid, Noviembre de 2019
Sobresaliente Cum Laude por Unanimidad (Mención Internacional)
- Nuevas estrategias para la prevención y control de la peste porcina africana
Lina Mur Gil
Facultad de Veterinaria de la Universidad Complutense de Madrid, Octubre 2014
Sobresaliente Cum Laude por Unanimidad (Mención Europea)
- Nuevas aportaciones al diagnóstico serológico y molecular de la peste porcina africana
Carmina Gallardo
Facultad de Ciencias de la Universidad Autónoma de Madrid, Junio 2003
Sobresaliente Cum Laude
- Nuevas aportaciones al estudio de la patogenia de la peste porcina africana
Julia Martín Fernández
Facultad de Veterinaria de la Universidad Complutense de Madrid, 1992
Apto Cum Laude
- Evolución de la respuesta humoral en animales infectados con el virus de la peste porcina africana
Olga Hortigüela Martos
Facultad de Biológicas de la Universidad Complutense de Madrid, 1990
Apto Cum Laude
- Nuevas aportaciones en el estudio de la respuesta humoral frente al virus de la peste porcina africana
María Luisa Arias Neira
Facultad de Ciencias de la Universidad Autónoma de Madrid, 1989
Apto Cum Laude
- Inmunoregulación del virus de la peste porcina africana
Silvia González Rebollar
Facultad de Veterinaria de la Universidad Complutense de Madrid, 1989
Apto Cum Laude
- Estudio inmunohistológico de los órganos linfoides mediante anticuerpos monoclonales en la peste porcina africana
Isabel Mínguez Tudela
Facultad de Veterinaria de la Universidad Complutense de Madrid, 1987
Apto Cum Laude
Indexed publications
- Ito S., Bosch J., Jurado C., Sanchez-Vizcaino JM., Isoda N. "Risk Assessment of African Swine Fever Virus Exposure to Sus scrofa in Japan Via Pork Products Brought in Air Passengers` Luggage". Pathogens, 9(4):302. 04/2020. (A)
- Cadenas-Fernández E., Sanchez-Vizcaino JM., Kosowska A., Rivera B., Mayoral-Alegre F., Rodriguez-Bertos A., Yao J., Bray J., Lokhandwala S., Mwangi W., Barasona JA. "Adenovirus-vectored African Swine Fever Virus Antigens Cocktail Is Not Protective against Virulent Arm07 Isolate in Eurasian Wild Boar". Pathogens, 9(171):1-14. 02/2020. (A)
- Cadenas-Fernández E., Sanchez-Vizcaino JM., Pintore A., Denurra D., Cherchi M., Jurado C., Vicente J., Barasona JA. "Free-Ranging Pig and Wild Boar Interactions in an Endemic Area of African Swine Fever". Frontiers in veterinary science, 6:376. 10/2019. (A)
- Jurado C., Mur L., Perez-Aguirreburualde MS., Cadenas-Fernández E., Martinez-Lopez B., Sanchez-Vizcaino JM., Perez A. "Risk of African swine fever virus introduction into the United States through smuggling of pork in air passenger luggage". Scientific reports, 9(1):14423. 10/2019. (A)
- Barasona JA., Gallardo C., Cadenas-Fernández E., Jurado C., Rivera B., Rodriguez-Bertos A., Arias M., Sanchez-Vizcaino JM. "First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II". Frontiers in veterinary science, 6(137):1-10. 04/2019. (A)
- Laddomada A., Rolesu S., Loi F., Cappai S., Oggiano A., Madrau MP., Sanna ML., Pilo G., Bandino E., Brundu D., Cherchi S., Masala S., Marongiu D., Bitti G., Desini P., Floris V., Mundula L., Carboni G., Pittau M., Feliziani F., Sanchez-Vizcaino JM., Jurado C., Guberti V., Chessa M., Muzzeddu M., Sardo D., Silvio B., Mulas D., Salis G., Zinzula P., Piredda S., De Martini A., Sgarangella F. "Surveillance and control of African Swine Fever in free-ranging pigs in Sardinia". Transboundary and Emerging Diseases, In Press, 66(3):1114-1119. 02/2019. (A)
- Cristina Jurado, Estefanía Cadenas Fernández, Sánchez-Vizcaíno JM. La peste porcina africana, la mayor amenaza del sector porcino mundial. Suis, ISSN 1699-7867, Nº. 156, 2019, págs. 12-18
- Arias M., Jurado C., Gallardo C., Fernandez-Pinero J., Sanchez-Vizcaino JM. "Gaps in African swine fever: Analysis and priorities". Transboundary and Emerging Diseases, 65 Suppl 1:235-247. 05/2018
- Jurado C., Martinez-Aviles M., De La Torre A., Stukelj M., de Carvalho Ferreira HC., Cerioli M., Sanchez-Vizcaino JM., Bellini S. "Relevant Measures to Prevent the Spread of African Swine Fever in the European Union Domestic Pig Sector". Front Vet Sci. 5:77. 4/2018.
- Jurado C., Fernández-Carrión E., Mur L., Rolesu S., Laddomada A., Sánchez-Vizcaíno JM. "Why is African swine fever still present in Sardinia?". Transbound Emerg Dis 65(2):557-566. 4/2018.
- Alkhamis MA., Gallardo C., Jurado C., Soler A., Arias M. y Sanchez-Vizcaino JM. "Phylodynamics and evolutionary epidemiology of African swine fever p72-CVR genes in Eurasia and Africa". PLoS ONE. 13(2):e0192565. 2/2018.
- Mur L., Sánchez-Vizcaíno J.M. Fernández-Carrión E., Jurado C., Rolesu s, Feliziani F., Laddomada A., Martínez-López B. "Understanding African Swine Fever infection dynamics in Sardinia using a spatially explicit transmission model in domestic pig farms". Transbound Emerg Dis. 65(1):e123-34. 2/2018.
- Arias M., Jurado C., Gallardo C., Fernandez-Pinero J., Sanchez-Vizcaino JM. "Gaps in African swine fever: Analysis and priorities". Transbound Emerg Dis. In press. 9/2017
- Bosch J., Rodriguez A., Iglesias I., Munoz MJ., Jurado C., Sanchez-Vizcaino JM. y de la Torre A. "Update on the Risk of Introduction of African Swine Fever by Wild Boar into Disease-Free European Union Countries". Transboundary and Emerging Diseases. 64(5):1424-1432. 10/2017
- Achenbach JE., Gallardo C., Nieto-Pelegrín E., Rivera-Arroyo B., Degefa-Negi T., Arias M., Jenberie S., Mulisa DD., Gizaw D., Gelaye E., Chibssa TR., Belaye A., Loitsch A., Forsa M., Yami M., Diallo A., Soler A., Lamien CE., Sánchez-Vizcaíno JM. "Identification of a New Genotype of African Swine Fever Virus in Domestic Pigs from Ethiopia". Transbound Emerg Dis. 64(5):1393-1404. 10/2017
- Fernandez-Carrion E, Martinez-Aviles M, Ivorra B, Martinez-Lopez B, Ramos AM, Sanchez-Vizcaino JM. "Motion-based video monitoring for early detection of livestock diseases: The case of African swine fever". PLoS One. 12(9):e0183793. 9/2017
- Mur L., Iscaro C., Cocco M., Jurado C., Rolesu S., De Mia GM., Feliziani F., Pérez-Sánchez R., Oleaga A., Sánchez-Vizcaíno JM. "Serological Surveillance and Direct Field Searching Reaffirm the Absence of Ornithodoros Erraticus Ticks Role in African Swine Fever Cycle in Sardinia". Transbound Emerg Dis. 64(4):1322-1328. 8/2017.
- Guinat C., Vergne T., Jurado-Diaz C., Sánchez-Vizcaíno JM., Dixon L., Pfeiffer DU. "Effectiveness and practicality of control strategies for African swine fever: what do we really know?". Vet Rec. 180(4):97. 1/2017.
- Mur L., Igolkin A., Varentsova A., Pershin A., Remyga S., Shevchenko I., Zhukov I., Sánchez-Vizcaíno, JM., “Detection of African Swine Fever Antibodies in Experimental and Field Samples from the Russian Federation: Implications for Control”. Transboundary and Emerging Diseases. 63(5):e436-40.10/2016.
- Giménez-Lirola LG., Mur L., Rivera B., Mogler M., Sun Y., Lizano S., Goodell C., Harris DL., Rowland RR., Gallardo C., Sánchez-Vizcaíno JM., Zimmerman J. "Detection of African Swine Fever Virus Antibodies in Serum and Oral Fluid Specimens Using a Recombinant Protein 30 (p30) Dual Matrix Indirect ELISA". PLoS One. 9;11(9):e0161230. 9/2016.
- Mur L., Atzeni M., Martínez-López B., Feliziani F., Rolesu S., Sanchez-Vizcaino JM. "Thirty-Five-Year Presence of African Swine Fever in Sardinia: History, Evolution and Risk Factors for Disease Maintenance". Transbound Emerg Dis. 63(2):e165-77. 4/2016.
- Pietschmann J., Mur L., Blome S., Beer M., Perez-Sanchez R., Oleaga A., Sanchez-Vizcaino JM. "African swine fever virus transmission cycles in Central Europe: Evaluation of wild boar-soft tick contacts through detection of antibodies against Ornithodoros erraticus saliva antigen". BMC Veterinary Research. 12(1):1. 1/2016
- De la Torre A, Bosch J, Iglesias I, Muñoz MJ, Mur L, Martínez-López B, Martínez M, Sánchez-Vizcaíno JM. "Assessing the Risk of African Swine Fever Introduction into the European Union by Wild Boar". Transbound Emerg Dis. 62(3):272-9. 6/2015.
- Sanchez-Vizcaino JM., Mur L., Bastos ADS., Penrith ML. "New insights into the role of ticks in African swine fever epidemiology". Rev Sci Tech Off Int Epiz. 34(2):503-511. 11/2015
- Nieto-Pelegrin E., Rivera B., Sanchez-Vizcaino JM. "First Detection of Antibodies Against African Swine Fever Virus in Faeces Samples". Transbound Emerg Dis. 62(6):594-602. 12/2015
- Gallardo C., Soler A., Nieto R., Sanchez MA., Martins C., Pelayo V., Carrascosa AL., Revilla Y., Simon A., Briones V., Sanchez-Vizcaino JM., Arias M. "Experimental Transmission of African Swine Fever (ASF) Low Virulent Isolate NH/P68 by Surviving Pigs". Transbound Emerg Dis. 62(6):612-622. 12/2015
- Martinez-López, B., M. Perez, A., Feliziani, F., Rolesu, S., Mur, L., Sánchez-Vizcaíno, JM., “Evaluation of the risk factors contributing to the African swine fever occurrence in Sardinia, Italy” frontiers in Microbiology, 314(6):1-10. 4/2015.
- Sanchez-Vizcaino JM., Mur L., Gomez-Villamandos JC., Carrasco L. "An Update on the Epidemiology and Pathology of African Swine Fever". Journal of Comparative Pathology. 152(1):9-21. 1/2015.
- Mur L., Martínez-López B., Costard S., de la Torre A., Jones BA., Martínez M., Sánchez-Vizcaíno F., Muñoz MJ., Pfeiffer DU., Sánchez-Vizcaíno, JM., Wieland B. "Modular framework to assess the risk of African swine fever virus entry into the European Union". BMC Vet Res. 10(1):145. 7/2014
- N. LeBlanc, M. Cortey, J. Fernandez Pinero, C. Gallando, Mesembe, A. R Okurut, L. Hearh, J.van Heerden, J.M. Sánchez-Vizcaíno, K. Stahl and S. Belák. ”Development of a Suspension Microarray for the Genotyping of African Swine Fever Virus Targeting the SNPs in the C-Terminal End of the p72 Gene Region of the Genome”. Transboundary and Emerging Diseases. 60(4):378-383. 8/2013.
- .Costard, S., Anne Jones, B., Martínez-López, B., Mur L., de la Torre, A., Martínez, M., Sánchez-Vizcaíno, F., Sánchez-Vizcaíno, J.M., Udo Pfeiffer, D., Wieland, B.,(2013) “Introduction of African Swine Fever into the European Union through ilegal Importation of Pork and Pork Products”. PLoS ONE 8(4): e61104. 4/2013.
- Mur, L., Gallardo, C., Soler, A., Zimmermman, J., Pelayo, V., Nieto, R., Sanchez-Vizcaino, JM., Arias, M. (2013) "Potential use of oral fluid samples for serological diagnosis of African swine fever". Veterinary Microbiology. 165(1-2):135-9. 7/2013.
- Costard, S., Mur, L., Lubroth, J., Sanchez-Vizcaino, JM., Pfeiffer, DU. (2013) "Epidemiology of African Swine Fever Virus". Virus Res. 173(1):191-7. 4/2013
- Sánchez-Vizcaíno, J.M., Mur L., Martínez-López B., (2013) “African swine fever (ASF): Five years around Europe”. Veterinary Microbiology 26;165(1-2):45-50. 7/2013.
- Mur, L., Martínez-López, B., Gallardo, C., Gortazar, C. Sánchez-Vizcaíno, JM. (2012) "Monitoring of African Swine Fever in the Wild Boar Population of the Most Recent Endemic Area of Spain". Transboundary and Emerging Diseases. 59(6):526-31. 12/2012.
- Sánchez-Vizcaíno, JM., Mur, L., and Martínez-López, B. (2012) "African Swine Fever: An Epidemiological Update". Transboundary and Emerging Diseases. Volume 59, Issue Supplement s1: 27-35. 3/2012.
- Mur, L., Martínez-López, B., Sánchez-Vizcaíno, JM. (2012) "Risk of African swine fever introduction into the European Union through transport-associated routes: returning trucks and waste from international ships and planes". BMC Vet Res. 8:149. 8/2012.
- Mur, L., Martinez-Lopez, B., Martinez, M., Costard, S., Wieland, B., Pfeiffer, DU. Sanchez-Vizcaino, JM. (2012). "Quantitative Risk Assessment for the Introduction of African Swine Fever Virus into the European Union by Legal Import of Live Pigs". Transboundary and emerging diseases. 59(2):134-44.4/2012.
- Gallardo, C.; Blanco, E.; Rodríguez, MJ.; Carrascosa, A.; Sanchez-Vizcaino. JM. (2006). Antigenic properties and diagnostic potential of African swine fever virus protein pp62 expressed in insect cells. J. Clin Microb 44, (3) 1489-1495
- Aguero, M.; Fernández, J.; Romero, L.; Sánchez, C.; Arias, M.; Sánchez-Vizcaíno, JM. (2003). Highly sensitive PCR assay for routine diagnosis of African swine fever virus in clinical samples. J. Clinical Microbiology 41 (9) 4431-4434.
- Blanco, E.; Rodríguez, J.; Carrascosa, A.; Sánchez-Vizcaíno, JM. (2000). Application of recombinant proteins p32, p54, pp62, and p10 to the development of improved methods for the diagnosis of African swine fever disease. Journal of Clinical Virology, 18: 159.
- Rodríguez, F.; Martín de las Muelas, J.; Herraez, P.; Sánchez-Vizcaíno, JM.; Fernández, A. (1996). Immunohistopathological studies of African swine fever (Strain E-75) infected bone marrow. J. Comp. Path. 114, 399-406.
- Bech-Nielsen. S.; Fernández, J.; Martínez-Pereda, F.; Espinosa, J.; Pérez Bonilla, Q.; Sánchez-Vizcaíno, JM. (1995). A case study an Outbreak of African swine fever in Spain. Br. Vet. Journal. 151(2), 203-214.
- Mebus, C.A.; House, C.; Ruiz Gonzalvo, F.; Pineda, J.M.; Tapiador, J.; Pire, J.J.; Bergada, J.; Yedloutschnig, R.J.; Sahu, S.; Becerra, V. and Sánchez-Vizcaíno, J.M. (1993). Survival of Foot-and-mouth disease, African swine fever, and Hog cholera viruses in spanish serrano cured hams and Iberian cured hams, shoulders and loins. Food Microbiology 10, 133-143.
- Arias, M.; Escribano, J.M.; Sánchez-Vizcaíno, J.M. (1993). Persistence of African swine fever antibody reactivity on ELISA and immunoblotting assays. Veterinary Record 133, 189-190.
- Bech-Nielsen, S.; Pérez Bonilla, Q.; Sánchez-Vizcaíno, J.M., (1993). Benefit-cost analysis of the current African swine fever erradication program in Spain and of an accelerated program. Preventive Veterinary Medicine, 17, 235-249.
- Bech-Nielsen, S.; Arias, M.L.; Panadero, J.; Escribano, J.A.; Gomez-Tejedor, C.; Perez. Q.; Sánchez-Vizcaíno, J.M. (1993). Laboratory diagnosis and disease occurrence in the current African swine fever eradication program in Spain, 1981-1991. Preventive Veterinary Medicine, 17, 225-234.
- González-Juarrero, M.; Lunney, J.K.; Sánchez-Vizcaíno, J.M.; Mebus, C. (1992). Modulation of splenic macrophages, and swine leukocyte antigen (SLA) and viral antigen expression following african swine fever virus (ASFV) inoculation. Arch. Virol. 123, 145-156.
- Fernández, A.; Perez, J.; Carrasco, L., Sierra, M.A.; Sánchez-Vizcaíno, J.M.; Jover, A. (1992). Detection of African swine fever viral antigens in paraffinembedded tissues by use of immunohistologic methods and polyclonal antibodies. Am. J. Vet. Med. 53 (8), 1462-1467.
- Fernández, A.; Pérez, J.; Carrasco, L.; Bautista, M.J.; Sánchez-Vizcaíno, J.M.; Sierra, M.A. (1992). Distribution of ASFV antigens in pig tissues experimentally infected with two different spanish virus isolates. J. Vet. Med. 39, 393-402.
- Martín-Fernández, J.; Igual, A.; Rueda, A.; Sánchez-Vizcaíno, J.M.; Alonso-Martí, C. (1991). Glomerular pathology in surviviring pigs experimentall, infected with ASF virus. Histol. Histopath 6, 115-121.
- González, S.; Mendoza, C.; Sánchez-Vizcaíno, J.M.; Alonso, F. (1990). Inhibitory effect of African swine fever virus on lectin-dependent swine lymphocyte proliferation. Veterinary Immunology and immunopathology, 26. 71-80.
- Pastor, M.J.; Laviada, M.D.; Sánchez‑Vizcaíno, J.M.; Escribano, J.M. (1989). Detection of African swine fever antibodies by immunoblotting assay. Can. J. Vet. Res. 53: 105‑107.
- Sierra, M.A.; Quezada, M.; Fernández, A.; Carrasco, L.; Gómez‑Villamandos, J.; Martín, J.; Sánchez‑Vizcaíno, J.M. (1989). Experimental African Swine Fever: Evidence of the virus in Intersticial Tissues of the Kidney. Vet. Pathol. 26: 173‑176.
- Mínguez, I.; Rueda, A.; Domínguez, J.; Sánchez‑Vizcaíno, J.M. (1988). Double labeling immunohistological study of african swine fever virus infected spleen and lymph nodes. Veterinary Pathology. 25: 193‑198.
- Wardley, R; Andrade, M; Black, D; Castro, F; Enjuanes, L; Hess, W; Mebus, C; Ordás, A; Rutilli, D; Sánchez‑Vizcaíno, J.M; Vigario, J; Wilkinson, P. (1983). African Swine Fever: Brief Review. Archives of Virology 76, 73‑90.
- Sánchez‑Vizcaíno, J.M; Slauson, D; Ruiz, F; Valero, F. (1981). Lymphocyte function and Cell Mediated Immunity in Pig infected with Experimental African Swine Fever. American Journal Veterinary Research 42 (8), 1335‑1341.
- Slauson, D; Sánchez‑Vizcaíno, J.M; (1981). Leukocyte ‑ dependent platelet vasoactive amine release and immune complex deposition in African Swine Fever. Veterinary Pathology 18, 813‑826.
Outreach publications
- Jurado C., Cadenas-Fernández E., Sanchez-Vizcaino JM. "Peste porcina africana: situación actual y perspectivas de futuro". Sólo cerdo ibérico, 41, Aeceriber. 2019. (Outreach arcticle)
- Barasona JA., Gallardo C., Cadenas-Fernández E., Jurado C., Rivera B., Rodriguez-Bertos A., Arias M., Sanchez-Vizcaino JM. "Vacunación oral en jabalí. Una nueva esperanza frente a la peste porcina africana". Porcinews, 19:36-45, Grupo comunicación Agrinews, S.L. 2019. (Outreach arcticle)
- Arias M., de la Torre A., Dixon L., Gallardo C., Jori F., Laddomada A., Martins C., Parkhouse M., Revilla Y., Rodriguez F., Sanchez-Vizcaino JM. "Approaches and Perspectives for Development of African Swine Fever Virus Vaccines". Vaccines. 10/2017
- Giménez-Lirola, LG., Mur, L., Rivera, B., Lizano, S., Goodell, C., Rowland, R., Mogler, M., Harris, DL., Gallardo, C., Arias, M., Sánchez-Vizcaíno, JM., Zimmerman, J.(2014). “Simultaneous detection of African swine fever virus antibodies in serum and oral fluid using a recombinant p30 antibody ELISA”. 57th AAVLD/118th USAHA Meeting. Kansas City, Missouri,October 16-22
- Sánchez-Vizcaíno, JM., Mur, L., Sánchez-Matamoros, A., Martínez-López B. (2014). “Peste porcina Africana: Nuevos retos y medidas para evitar su propagación”. 82ª Sesión General. Asamblea Mundial. Organización mundial de sanidad animal. París 25-30 de mayo.
- Sánchez-Vizcaíno JM., Mur L., Sánchez-Matamoros A., Martínez-López B. (2014) "African Swine Fever: New challenges and measures to prevent its spread". 82nd General Session OIE. World Assembly. Paris. Mayo.
- Sánchez-Vzcaíno, J.M., Mur. L. (2012) “Peste porcína africana: un riesgo remergente procedente del Este de Europa”. Boletin OIE. Nª2012-4, pp.75-76.
- A.D. Zaberezhny. T.I Aliper, T.V Grebennikova, O.A. Verkhovsky, Sánchez-Vizcaíno JM, Mur, L., E.A. Nepoklonov, anda D.K. Lvov. (2012) “African Swine Fever in Russian Federation”. Problems of virology 5-2012, pp-4-10.
- Mur, L., Sánchez-Vizcaíno JM. (2012) "Peste porcina africana. Reconocer la enfermedad en campo". 3tres3.com. 30/3/2012
- Sánchez-Vizcaíno JM., Mur, L. (2012). "La PPA: Breve visión desde el pasado, presente y... futuro?". 3tres3.com. 2012/1/24.
- Mur, L., Martínez-López, B., Sánchez-Vizcaíno, JM (2011) “African swine fever: are we aware?” Pig progress. The international magazine on pig production. Vol.27. pp7-9.
- Mur, L., Martínez-López, B., Martínez-Avilés, M., Sánchez-Vizcaíno, JM. (2010). “African Swine Fever: Import of live pigs not a high risk”. Pig Progress, IPVS Focus, 20.
- Sánchez-Vizcaíno, JM. (2010). “La peste porcína africana”. Albéitar, 141, 22-24.
- Mur-Gil, L., Martínez-López, B., Sánchez-Vizcaíno, JM. (2009).” El despertar de la Peste Porcina Africana”. Revista Complutense de Ciencias Veterinarias. 3 (2) 149-157.
- Martínez-López, B., Perez, A., Sánchez-Vizcaíno, JM (2009). “Evaluation of the potential spread and effectiveness of control measures for Classical Swine Fever into Spain by using a spatial and stochastic model”. Proceeding, ISVEE XII.
- Hortiguela, O.; Sánchez-Vizcaíno, JM.; Arias, M. (1993). Quantification of the antibody response in african swine fever infection. Adaptation of an ELISA using monoclonal antibodies against different isotypes of immunoglobulins. Inv. Agr.: prod. Sanid anim. 8 (3), 289-298.
- Pastor, M.J.; Sánchez‑Vizcaíno, J.M.; Escribano, J.M. (1988). Dos nuevas técnicas para el diagnóstico de la peste porcina africana: Inmunoelectrotransferencia y enzimo‑inmuno‑adsorción. Med. Vet. 5, 275‑292.
- Sánchez-Vizcaíno, JM. (1984). Estudios de inmunidad de base celular e inmunopatología en la peste porcina africana. Ed. Universidad Complutense. M 4036-1984.
- Sánchez-Vizcaíno, J.M; Crowther, J; Wardley, R. (1983). A collaborative study on the use of the ELISA in the diagnosis of ASF. African Swine Fever. CEC. Eur. 8466, 297‑325.
- Sánchez-Vizcaíno, J.M; Tabares, E; Salvador, E; Ordás, A. (1982). Comparative Studies of two antigens for the use in the Indirect Elisa test for the detection of ASF antibodies. African Swine Fever. CEC Eur. 8466, 101‑106.
- Sánchez-Vizcaíno, J.M; Salvador, E; Mesanza, R; Sánchez Botija, C. (1980). Enzimoinmunoensayo (ELISA): Un nuevo método de diagnóstico para la peste porcina africana. ONE 9, 18‑23.
- Sánchez-Vizcaíno, J.M; Martín, L; Ordás, A. (1979). Adaptación y evaluación del enzimoinmunoensayo para la detección de anticuerpos de peste porcina africana. Laboratorio 67, (400) 311‑319.


